Dr. ZHU Jian and Professor WU Yongling, published a paper entitled “Microstructures, wear resistance and corrosion resistance of CoCrFeNi high entropy alloys coating on AZ91 Mg alloy prepared by cold spray” (10.1016/j.jallcom.2022.166698) in Journal of Alloys and Compounds.
In this work, a fully dense CoCrFeNi high entropy alloys (HEAs) coating with homogeneous microstructurewas successfully prepared on AZ91 alloy by cold spray. It is innovative to cross-integrate HEAs with Mgalloys using cold spray to break through the “bottleneck” of Mg alloys. The average microhardness of thecoating was 5 times higher than Mg alloys. The weight loss of the coating in wear test was 2 orders ofmagnitude lower than Mg alloys. The Ecorr of the coating was noticeably improved to be ~ - 290 mVSCE andthe icoor of the coating was significantly decreased by 4–5 orders of magnitude. The coating exhibitedsignificantly spontaneous passivation due to the multi-layer passive film composed of FeO, Cr2O3 and NiO.The weight loss of CoCrFeNi coated AZ91 plates was below 1% after 28 days immersion. Lots of tiny micropits in size less than 1 µm were distributed on the corroded coating surface because the formation of largepitting pits was restricted. Unique corrosion mechanisms of the coatings were found: the repeatedly repassivation effect and the competing effect triggered by the rapid initiations of new pittings. The stablepassive film and uniform microstructure of the coating resulted in the significant decrease of corrosion rate.The shielding effect of the coating can bring outstanding surface protection for Mg alloys.
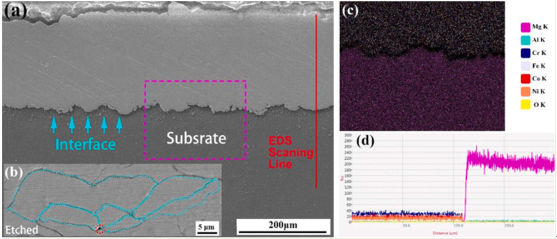
Fig 1. The SEM images of the vertical cross-section of the (a) polished and (b) etched coating on AZ91; EDS (c) map and (d) line scanning image of the interface
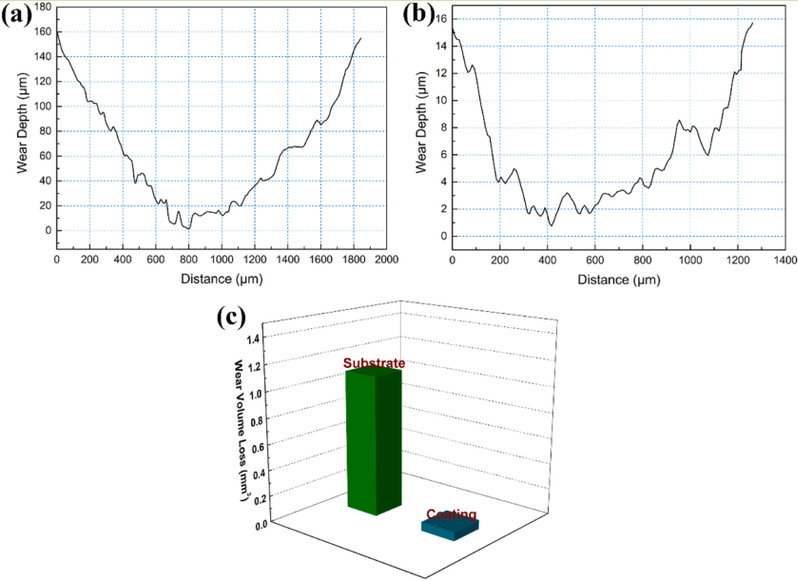
Fig 3. The cross-sectional profiles of the worn tracks of (a) the substrate and (b) coating; (c) the weight loss of the substrate and coating.
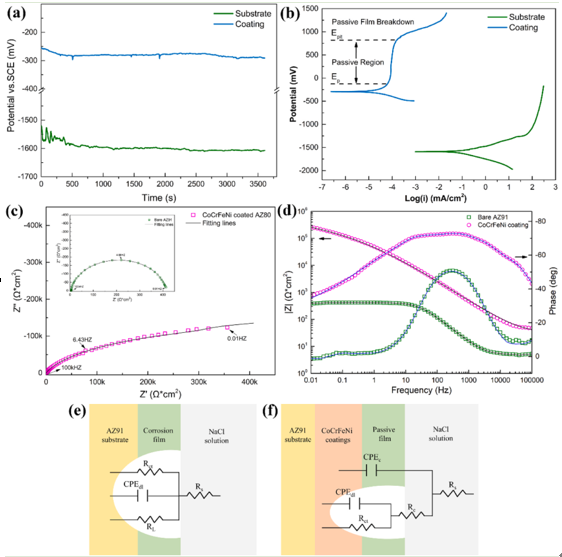
Fig 4. Electrochemical test results of the bare and coated Mg alloys in NaCl solution: (a) OCP and (b) PDP curves; (c) Nyquist and (d) Bode plots; Equivalent electrical circuits of the (e) substrate and (f) coating.


